 |
 |
- Search
| J Electrodiagn Neuromuscul Dis > Volume 24(3); 2022 > Article |
|
Abstract
Objective
We investigated the factors affecting electrodiagnostic (EDX) parameters after carpal tunnel release (CTR).
Methods
Thirty-nine cases with clinically diagnosed carpal tunnel syndrome who received CTR and EDX studies before and after CTR were enrolled in this study. We analyzed EDX parameters such as distal onset latency and the amplitude of median compound motor action potentials (CMAPs) and sensory nerve action potentials (SNAPs).
Results
Among 39 cases, 24 (61.5%) showed improvement of at least 1 grade, based on Blandâs scale, after CTR. Follow-up EDX studies were performed 6 to 36 months after CTR. Improvement on Blandâs scale was shown in 50% of patients who received follow-up EDX studies at 6 and 12 months after CTR and in all patients who received follow-up at 24 and 36 months. The EDX parameters showed significant recovery. Younger patients showed greater recovery of SNAP amplitude (p = 0.021, r = -0.369) after CTR. The preoperative severe group showed greater recovery of CMAP (both amplitude and latency) than the non-severe group (p = 0.011 and p = 0.038, respectively).
Carpal tunnel syndrome (CTS) is the most common focal entrapment mononeuropathy, affecting 1% of the population [1,2]. When patients do not improve with conservative treatment, carpal tunnel release (CTR) surgery is frequently performed. Approximately 70% to 90% of patients have good to excellent long-term outcomes with CTR [3]. The remaining patients have poor outcomes, which can be classified into one of 3 categories: persistent, recurrent, or new symptoms [4]. An understanding of the factors that predict a poor outcome after CTR would be beneficial during preoperative counseling and provide more accurate expectations for postoperative outcomes.
Many existing studies on CTS have reported its risk factors, along with methods for its diagnosis and treatment; however, few have investigated the factors affecting the postoperative improvement of CTS. Even in studies that identified predictors of postoperative outcomes, the results were inconsistent. Previous studies have found that worse outcomes of CTR have been associated with preoperative variables such as preoperative muscle weakness or atrophy [5-7], predisposing medical conditions [6], including diabetes and thyroid disease [8], heavy or repetitive manual work [9,10], and exposure to vibration [11]. However, other studies have found that predisposing medical comorbidities [12-18], patient factors (age, sex) [19,20], duration of symptoms before surgery [19], neurophysiologic testing [19,21], physical examination findings [22], and body mass index [23] did not affect the postoperative outcomes. The severity of nerve conduction or electromyographic abnormalities is generally not associated with surgical outcomes [6,7,9,10].
Most previous studies have evaluated postoperative improvements in CTS through patient-reported symptom relief and satisfaction [24-30]. There have been few comparative studies on electrodiagnostic (EDX) studies before and after CTR in the same patients. Our study intended to analyze changes in EDX parameters after CTR instead of patient-reported symptom relief. The aim of this study was to describe the quantitative changes in EDX parameters after CTR and to clarify the factors affecting the recovery of the EDX parameters. Our hypothesis was that older age, longer durations of symptoms, and greater preoperative severity might negatively affect EDX recovery. An understanding of the factors that predict postoperative outcomes would be beneficial during preoperative counseling and provide more accurate expectations for the prognosis.
This retrospective study was approved by the ethics committee of Chung Ang University Hospital (approval number: 2109-029-19386) and was performed in accordance with the Declaration of Helsinki.
We retrospectively screened patients who visited an EDX laboratory affiliated with the Department of Physical Medicine and Rehabilitation of a single tertiary hospital from January 2012 to January 2022 and who presented with hand tingling. Patients were eligible if they met all of the following criteria: (1) they were diagnosed with CTS based on an EDX study, (2) they received CTR by a single orthopedic surgeon at the same hospital, and (3) they received a follow-up EDX study after CTR.
Patients were excluded if (1) they had diabetes before CTR; (2) they had no medical records concerning their medical comorbidities, including diabetes and duration of symptoms at the time of CTR; (3) their EDX results suggested combined cervical myeloradiculopathy or ulnar neuropathy, generalized demyelinating polyneuropathies, generalized axonal neuropathy related to end-stage renal disease, or trauma-related median neuropathy; or (4) they were on hemodialysis. A total of 39 cases in 35 patients were enrolled in this study.
We expected that age, sex, duration of symptoms, and the severity of findings on the preoperative EDX study might affect the results of the follow-up EDX studies. We surveyed these factors through a review of medical records.
The performance of CTR was dependent on the surgeonâs decision and based on each patientâs preoperative severity, duration of symptoms, previous history of treatments, comorbidities, and other factors.
The EDX studies were performed using a Nicolet EDX EMG system (Natus, Pleasanton, CA, USA). In our EDX laboratory, the patientâs skin temperature was routinely maintained between 31°C and 34°C. In nerve conduction studies (NCSs), the conventional surface electrode technique was used. The active recording electrode was placed on the belly of the abductor pollicis brevis muscle, and the reference electrode was attached distally to the insertion of the muscle during the motor NCSs. Wrist stimulation was performed 8 cm proximal to the active recording electrode. The onset latency (CMAPlatency) and baseline to peak amplitude (CMAPamplitude) values of the compound motor action potential (CMAP) were measured. Antidromic sensory nerve conduction was recorded from the index finger and stimulated in the wrist. The distance between the recording electrode and stimulator was 14 cm. Of the sensory nerve action potentials (SNAPs), the onset latencies (SNAPlatency) and baseline to peak amplitudes (SNAPamplitude) were measured.
In EDX studies, CTS was diagnosed when one or more of the following criteria were satisfied: (1) When the median CMAPlatency was 4.2 ms or more, (2) When the median SNAPlatency was 3.2 ms or more [31,32].
Additive NCS of other nerves and needle electromyography ruled out CTS-mimicking diseases such as cervical radiculopathy, motor neuron disease, and brachial plexopathy.
The degree of the patientâs median nerve damage was classified into grades 1 to 6 according to Blandâs neurophysiologic grading scale (Table 1) [33]. The patientâs preoperative severity was classified as severe (Blandâs scale 4-6) or non-severe (Blandâs scale 1-3). Improvement was defined as at least a 1-grade positive change on Blandâs scale after CTR. We also evaluated the recovery of EDX parameters by measuring the differences (ÎCMAPamplitude, ÎCMAPlatency, ÎSNAPamplitude, and ÎSNAPlatency) between the preoperative and postoperative CMAPamplitude, CMAPlatency, SNAPamplitude, and SNAPlatency values.
Statistical analyses were performed using IBM SPSS ver. 21.0 (IBM Corp., Armonk, NY, USA). The paired t-test was used to compare the differences between the preoperative and postoperative ÎCMAPamplitude, ÎCMAPlatency, ÎSNAPamplitude, and ÎSNAPlatency values. The Mann-Whitney U-test and chi-square tests were used to identify significant variables in the univariate analysis of improvement according to Blandâs scale. Spearmanâs correlation analysis was performed to examine the correlation between changes in EDX parameters (ÎCMAPamplitude, ÎCMAPlatency, ÎSNAPamplitude, and ÎSNAPlatency) and continuous variables (age and duration of symptoms). Point-biserial correlation analysis was conducted to assess the correlation between changes in EDX parameters and categorical variables (sex and preoperative severity).
All statistical tests were conducted at a two-sided 5% significance level, and all reported p-values were two-sided. Statistical significance was set at p < 0.05.
In total, 39 CTS cases from 35 patients were included in this study. Four patients had bilateral CTS and received bilateral CTR. The clinical characteristics of patients are summarized in Table 2. The mean age of the patients was 56.3 Âą 8.7 years, and the mean duration of symptoms at the first visit was 27.8 Âą 38.7 months. Four cases (10.3%) were in men and 35 cases (89.7%) were in men. Fifteen cases (38.5%) were on the right, 16 cases (41.0%) were on the left, and 4 cases were on both sides (Table 2).
Based on Blandâs scale, 5 cases (12.8%) were classified as grade 2 (mild), 20 cases (51.3%) were grade 3 (moderately severe), 2 cases (5.1%) were grade 4 (severe), 7 cases (17.9%) were grade 5 (very severe), and 5 cases (12.8%) were grade 6 (extremely severe). Patients were classified into 2 subgroups according to their preoperative severity: severe (Blandâs scale 4-6) and non-severe group (Blandâs scale 1-3). The severe group accounted for 14 cases (35.9%) and the non-severe group accounted for 25 cases (64.1%). The subgroups did not show significant differences in terms of the sex ratio (p = 0.123). However, there were significant differences in age and duration of symptoms between the 2 groups (age, p = 0.006; duration of symptoms, p = 0.028) (Table 3).
Postoperative follow-up EDX studies were performed in 20 cases (51.3%) at 6 months, 10 cases (25.6%) at 12 months, 6 cases (15.4%) at 24 months, and 3 cases (7.7%) at 36 months.
There was significant recovery of EDX parameters, such as ÎCMAPamplitude, ÎCMAPlatency, ÎSNAPamplitude, and ÎSNAPlatency (ÎCMAPamplitude, p = 0.024; ÎCMAPlatency, p < 0.001; ÎSNAPamplitude, p < 0.001; and ÎSNAPlatency, p < 0.001) after CTR (Table 4).
Even in the cases with âno responseâ on the preoperative EDX studies, the recovery of obtainable EDX responses after CTR was reported in 25.0% of cases (1 of 4) for CMAP and 50.0% of cases (6 of 12) for SNAP, respectively.
We analyzed improvement patterns according to casesâ preoperative severity based on Blandâs scale. Of the 39 cases, 24 cases (61.5%) showed improvements (at least a 1-grade positive change on Blandâs scale) after CTR, and 15 cases (38.5%) showed non-improvement.
The final change in each caseâs Blandâs scale after CTR is presented in Fig. 1A. The line with a slope of 1 presents cases with the same Blandâs scale before and after CTR. The cases below the line are those in which improvement occurred, while those above and at the line comprise non-improvement cases. Improvement was observed in (60%) out of 5 cases with preoperative Blandâs scale 2, 12 (60%) out of 20 cases with preoperative Blandâs scale 3, one (50.0%) out of 2 cases with preoperative Blandâs scale 4, 6 (85.7%) out of 7 cases with preoperative Blandâs scale 5, 2 (40%) out of 5 cases with preoperative Blandâs scale 6 (Fig. 1A). The overall patients were classified according to the timing of the follow-up EDX study. Follow-up EDX studies were performed 6 months to 36 months after CTR. Ten (50.0%) of 20 patients who received a follow-up EDX study at 6 months after CTR showed improvement on Blandâs scale (Fig. 1B). Five (50.0%) of 10 patients who received follow-up at 12 months after CTR revealed improvement on Blandâs scale (Fig. 1C). All patients who received follow-up EDX at 24 (n = 6) and 36 months (n = 3) after CTR demonstrated improvement on Blandâs scale (Fig. 1D, E).
Univariate analysis revealed that improvements in Blandâs scale were not associated with any clinical factors (sex, p = 0.631; age, p = 0.875; duration, p = 0.598; preoperative severity, p = 1.000; and period of follow-up EDX study, p = 0.051). The improvement group received follow-up EDX studies on average 15.5 Âą 10.8 months after CTR, and the non-improvement group had follow-up examinations at an average of 8.0 Âą 2.9 months after CTR. Even though no statistically significant difference in the timing of follow-up EDX studies was found between the improvement group and the non-improvement group (p = 0.051), the follow-up period of the improvement group tended to be longer than that of the non-improvement group (Table 5).
The quantitative analysis of changes in EDX parameters after CTR showed a significant inverse correlation between age and ÎSNAPamplitude, suggesting that younger age was associated with greater recovery of ÎSNAPamplitude after CTR (p = 0.021, r = -0.369). A significant correlation was found between preoperative severity and recovery of both ÎCMAPamplitude and ÎCMAPlatency values after CTR, in which the preoperative severe group showed more recovery of ÎCMAPamplitude and ÎCMAPlatency values after CTR (ÎCMAPamplitude, p = 0.011, r = 0.403; ÎCMAPlatency, p = 0.038, r = 0.334) (Table 6).
Among 39 cases, 24 (61.5%) showed improvement in CTS (at least a 1-grade positive change on Blandâs scale) in the follow-up EDX study after CTR. This was similar to the 70% to 90% improvement rates reported in other studies [3,34]. There was a significant recovery of EDX parameters such as ÎCMAPamplitude, ÎCMAPlatency, ÎSNAPamplitude, and ÎSNAPlatency after CTR. No statistically significant associations were found between improvements on Blandâs scale and clinical factors (sex, age, duration of symptoms, and preoperative severity), whereas the quantitative analysis of EDX parameters suggested that age and preoperative severity can affect the recovery of EDX parameters after CTR.
This study aimed to describe quantitative changes in EDX parameters after CTR and to clarify the factors affecting the recovery of EDX parameters. We analyzed the association between improvement based on Blandâs scale and each clinical factor (sex, age, duration of symptoms, and preoperative severity), as well as correlations between the recovery of quantitative EDX parameters and clinical factors. A unique feature of our study was that surgical outcomes were quantified objectively by analyzing changes in EDX parameters instead of using patient-reported subjective symptoms and satisfaction. No statistically significant associations were found between improvements on Blandâs scale and clinical factors, whereas preoperative severity and age affected changes in quantitative EDX parameters. Younger patients showed greater recovery of ÎSNAPamplitude values after CTR. The degree of CMAP recovery was greater (for both ÎCMAPamplitude and ÎCMAPlatency) in the preoperative severe group. These results may be related to the relatively low granularity of Blandâs scale. For example, an improvement in ÎCMAPlatency values may not be reflected by a change on Blandâs scale.
Our results showed significant differences in age and duration of symptoms between the preoperative severe (Blandâs scale 4-6) and non-severe (Blandâs scale 1-3) groups. The mean age and duration of symptoms in the severe group were greater and longer than those in the non-severe group, respectively. Chronic entrapment may cause severe nerve damage, such as irreversible axonal damage during a long disease period, which can affect severity.
Our quantitative analysis of EDX parameters was consistent with our hypothesis that age can affect the recovery of EDX parameters after CTR, suggesting that younger patients showed greater recovery of ÎSNAPamplitude values after CTR. It is widely known that motor and sensory nerve conduction responses gradually decline with aging in the general population. Several studies also have reported a significant correlation between age and the course of entrapment neuropathy. Schwartz and Chan reported a progressive increase in distal motor latency and a progressive decrease in the CMAP amplitudes of older patients with CTS compared to younger patients; therefore, these older patients may require different therapeutic approaches [35]. Kim et al. [36] also compared NCS findings between younger and older groups and reported that CTS often has a progressive, non-remitting course in elderly patients aged over 60 years, possibly due to different mechanisms according to age. Based on the current evidence, elderly patients have less predictable symptomatic and functional improvements after CTR than younger patients [37]. These results are in line with our finding that EDX recovery after CTR was related to age.
Regarding the effect of preoperative severity on surgical outcomes, the recovery of CMAP after CTR was contrary to our hypothesis that patients with severe CTS would have worse outcomes. This result is probably due to the difference in the preoperative CMAPamplitude values between the severe and non-severe groups based on Blandâs scale. In our criteria for preoperative severity, the severe group (Blandâs scale 4-6) had relatively low CMAPamplitude values in the preoperative EDX studies, and there was substantial potential for recovery after CTR. In contrast, in the non-severe group (Blandâs scale 1-3), the preoperative CMAPamplitude values were nearly normal in most cases, suggesting a limited possibility for recovery after CTR. Although our results may have been influenced by the relatively low granularity of Blandâs scale, obtainable CMAP responses after CTR were reported in 25.0% (1 of 4 cases) of the âno responseâ cases in preoperative EDX studies. Reversible demyelination and nerve regeneration may be possible, even in extremely severe cases.
We expected that a longer duration of symptoms would be related to poorer EDX recovery. Debate continues regarding how symptom duration affects surgical outcomes. Some studies have suggested that a longer duration is associated with worse outcomes (i.e., less improvement in symptoms after surgery) [5,7,8,34]. Long-term exposure to entrapment can cause severe nerve damage, including irreversible axonal damage, leading to a poor prognosis. However, Porter et al. [19] reported that the preoperative durations of symptoms did not affect postoperative outcomes. Our study likewise revealed that symptom duration was not significantly associated with improvements in CTS based on both Blandâs scale and the quantitative analysis of EDX parameters after CTR, which was contrary to our hypotheses. We expected that chronicity might result in irreversible axonal loss, which therefore might lead to poor recovery, even with CTR. However, the pathological process of entrapment neuropathy can involve both axonal loss and reversible demyelination and remyelination [37]. Surgical decompression can be effective even in some cases of chronic advanced compressive neuropathy by enhancing the reversibility of the demyelination and axonal regeneration [37-42]. Therefore, surgery can be beneficial, regardless of the duration of symptoms.
Our results suggest that various clinical factors should be considered when making surgical decisions. Our quantitative analysis of the recovery of EDX parameters revealed that younger patients showed more recovery of ÎSNAPamplitude values. This finding suggests that age can affect EDX recovery after CTR and serve as a factor helping to determine whether surgery is indicated. Several studies have suggested that different therapeutic approaches are needed for older and younger patients, as these ages undergo different clinical courses via different mechanisms [35,36]. Elderly patients with CTS may develop severe motor impairment within a short period, so they should be promptly referred for surgical decompression [35]. However, our study also suggested that improvement after CTR occurred regardless of factors such as preoperative severity and duration of symptoms, although the severe group was relatively older. Patients with severe CTS also showed some improvement after CTR. Clinicians should decide on surgery with consideration of not only age, but also several other clinical factors.
Postoperative follow-up EDX studies were performed 6 to 36 months after CTR in previous several studies [43,44]. Interestingly, our study showed that 9 (37.5%) out of 24 cases that showed improvement received follow-up EDX studies 24 to 36 months after CTR. This result suggests that sustained EDX recovery is possible even more than 1 year after CTR. Although this trend was statistically marginal (p = 0.051), the interval of recovery after CTR tended to be longer in cases of improvement. A previous study reported that advanced CTS patients with severe axonal damage took considerably longer to recover after CTR, and several months of follow-up did not suffice to evaluate postoperative EDX recovery in these patients [42]. Our results also suggest that the EDX parameters might gradually recover after CTR over a long period of time, and serial follow-up studies for sufficient periods are recommended to determine the effects of CTR [42,44].
However, this study has some limitations. First, the sample size was relatively small. Second, this was a retrospective study. This study only analyzed the EDX recovery after CTR, and could not investigate the correlation between EDX recovery and clinical symptoms. Third, the period of the postoperative follow-up EDX studies was not standardized. Fourth, there may have been selection bias because not all patients underwent a follow-up EDX study after CTR. Fifth, the composition of the patients was quite heterogeneous, as the patients had many different reasons for receiving follow-up EDX studies after CTR. Further prospective studies are required to obtain more accurate outcome measures.
We confirmed the effectiveness of CTR through EDX studies regardless of preoperative EDX severity, the duration of symptoms, and sex. Our results suggest that the EDX parameters might gradually recover after CTR over a long period of time. Significant recovery of EDX parameters, including ÎCMAPamplitude, ÎCMAPlatency, ÎSNAPamplitude, and ÎSNAPlatency, was noted after CTR. No statistically significant associations were found between improvements in Blandâs scale and clinical factors, whereas the quantitative analysis of EDX parameters suggested that age and preoperative severity can affect the recovery of EDX parameters after CTR.
Fig. 1.
The changes in each patientâs Blandâs scale after carpal tunnel release (CTR) according to the timing of the follow-up electrodiagnostic (EDX) study. (A) Changes in Blandâs scale after CTR in all patients (n = 39). (B) Changes in Blandâs scale in patients who received a follow-up EDX study 6 months after CTR (n = 20). (C) Changes in Blandâs scale in patients who received a follow-up EDX study 12 months after CTR (n = 10). (D) Changes in Blandâs scale in patients who received a follow-up EDX study 24 months after CTR (n = 6). (E) Changes in Blandâs scale in patients who received a follow-up EDX study at 36 months after CTR (n = 3).
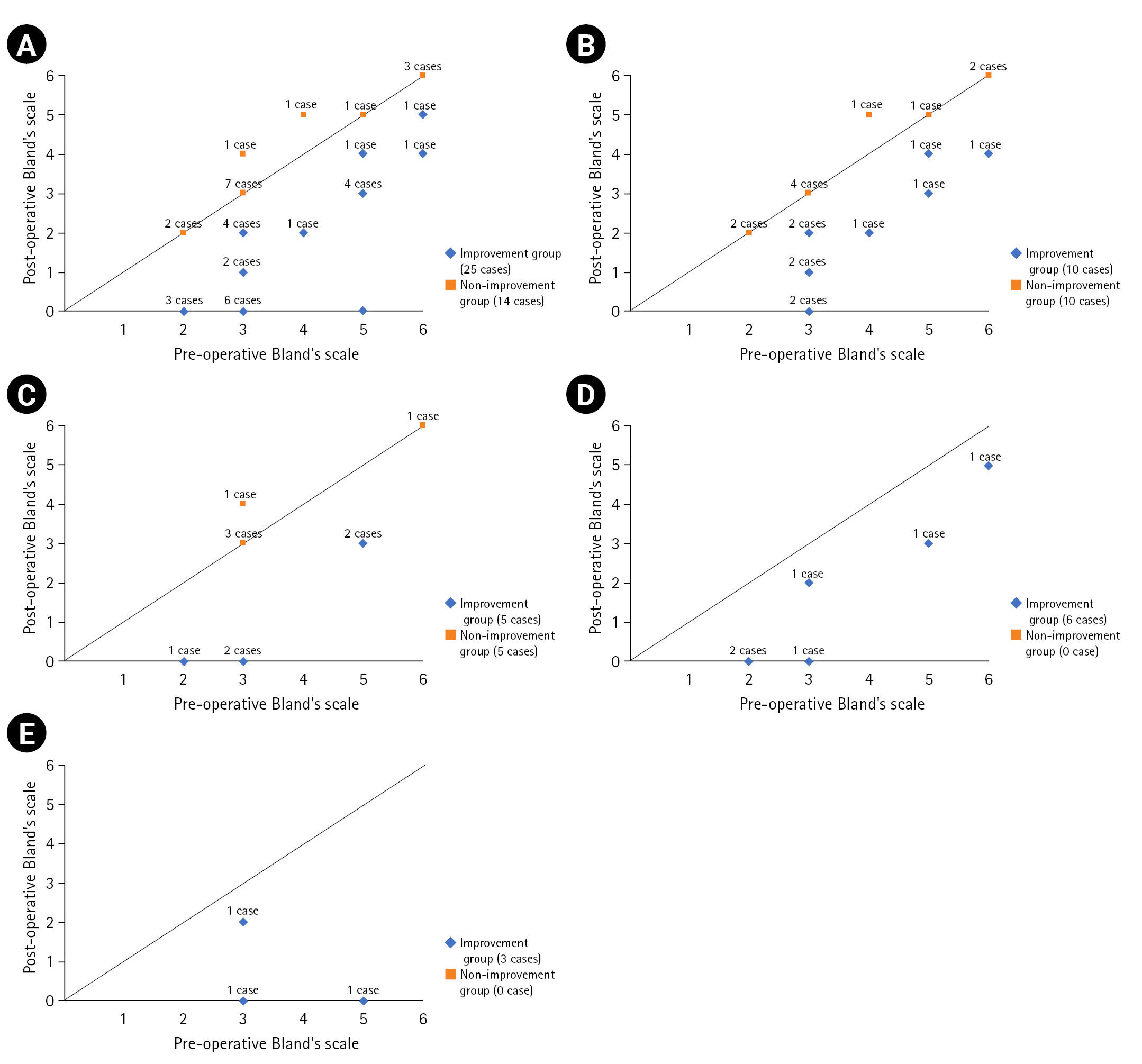
Table 1.
Blandâs Neurophysiologic Grading Scale for Carpal Tunnel Syndrome
EDX, electrodiagnostic; CTS, carpal tunnel syndrome; PWDSLD, palm wrist distal sensory latency difference; DML, distal motor latency; NCV, nerve conduction velocity; SNAP, sensory nerve action potential; CMAP, compound motor action potential; APB, abductor pollicis brevis muscle.
Based on [33]
Table 2.
Clinical Characteristics of the Study Population
Table 3.
Baseline Characteristics of the Subgroups according to Preoperative Severity
| Variable | Severe group* | Non-severe group | p-value |
|---|---|---|---|
| Number | 14 | 25 | |
| Sex (male:female) | 3:11 | 1:24 | 0.123 |
| Age (y) | 61.6 Âą 6.9 (52-70) | 53.3 Âą 8.3 (30-69) | 0.006â |
| Duration of symptoms (mo) | 46.0 Âą 50.4 (4-120) | 17.7 Âą 26.4 (1-120) | 0.028â |
Table 4.
Changes in Electrodiagnostic Parameters after Carpal Tunnel Release
| Parameter | Preoperative | Postoperative | Difference (Î) | p-value |
|---|---|---|---|---|
| CMAP amplitude (mV) | 7.5 Âą 4.4 | 8.3 Âą 4.5 | 0.8 Âą 2.1 | 0.024* |
| SNAP amplitude (ÂľV) | 10.4 Âą 11.8 | 17.2 Âą 13.4 | 6.8 Âą 9.1 | < 0.001* |
| CMAP latency (ms) | 6.1 Âą 3.0 | 4.8 Âą 2.6 | 1.2 Âą 1.9 | < 0.001* |
| SNAP latency (ms) | 4.9 Âą 1.5 | 3.8 Âą 1.4 | 1.1 Âą 1.2 | < 0.001* |
Table 5.
Analysis of Clinical Factors Affecting Improvement Based on Blandâs Scale after Carpal Tunnel Release
| Variable | Improvement group* | Non-improvement group | p-value |
|---|---|---|---|
| Number | 24 (61.5) | 15 (38.5) | |
| Sex | |||
| âFemale | 22 | 13 | 0.631 |
| âMale | 2 | 2 | |
| Age (y) | 56.4 Âą 8.4 | 56.0 Âą 9.4 | 0.875 |
| Duration of symptoms (mo) | 30.0 Âą 42.8 | 24.3 Âą 32.1 | 0.598 |
| Preoperative severity | |||
| âSevere group | 9 | 5 | 1.000 |
| âNon-severe group | 15 | 10 | |
| Timing of follow-up EDX study (mo) | 15.5 Âą 10.8 | 8.0 Âą 2.9 | 0.051 |
Table 6.
Analysis of Clinical Factors Affecting the Recovery of Electrodiagnostic Parameters After Carpal Tunnel Release
| Variable |
ÎCMAPamplitude |
ÎSNAPamplitude |
ÎCMAPlatency |
ÎSNAPlatency |
||||
|---|---|---|---|---|---|---|---|---|
| r | p-pvalue | r | p-value | r | p-value | r | p-value | |
| Age | -0.023 | 0.890 | -0.369 | 0.021* | 0.055 | 0.741 | -0.128 | 0.437 |
| Sex | 0.016 | 0.922 | 0.045 | 0.784 | 0.053 | 0.750 | 0.026 | 0.873 |
| Duration | 0.048 | 0.773 | -0.169 | 0.303 | 0.127 | 0.443 | 0.122 | 0.458 |
| Preoperative severity | 0.403 | 0.011â | -0.218 | 0.183 | 0.334 | 0.038â | 0.029 | 0.862 |
| Period of follow-up EDX study | -0.007 | 0.966 | 0.233 | 0.153 | 0.185 | 0.261 | 0.183 | 0.265 |
References
1. Wang L: Guiding treatment for carpal tunnel syndrome. Phys Med Rehabil Clin N Am 2018;29:751-760.


2. Levine DW, Simmons BP, Koris MJ, Daltroy LH, Hohl GG, Fossel AH, et al: A self-administered questionnaire for the assessment of severity of symptoms and functional status in carpal tunnel syndrome. J Bone Joint Surg Am 1993;75:1585-1592.


3. Brown RA, Gelberman RH, Seiler JG, Abrahamsson SO, Weiland AJ, Urbaniak JR, et al: Carpal tunnel release: a prospective, randomized assessment of open and endoscopic methods. J Bone Joint Surg Am 1993;75:1265-1275.


4. Patrick DL, Deyo RA: Generic and disease-specific measures in assessing health status and quality of life. Med Care 1989;27(3 Suppl):S217-S232.


5. Nau HE, Lange B, Lange S: Prediction of outcome of decompression for carpal tunnel syndrome. J Hand Surg Br 1988;13:391-394.


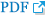
6. Kulick MI, Gordillo G, Javidi T, Kilgore ES, Newmayer WL: Long-term analysis of patients having surgical treatment for carpal tunnel syndrome. J Hand Surg Am 1986;11:59-66.


7. MĂźhlau G, Both R, Kunath H: Carpal tunnel syndrome: course and prognosis. J Neurol 1984;231:83-86.


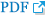
8. DeStefano F, Nordstrom DL, Vierkant RA: Long-term symptom outcomes of carpal tunnel syndrome and its treatment. J Hand Surg Am 1997;22:200-210.


9. Yu GZ, Firrell JC, Tsai TM: Pre-operative factors and treatment outcome following carpal tunnel release. J Hand Surg Br 1992;17:646-650.


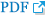
10. Al-Qattan MM, Bowen V, Manktelow RT: Factors associated with poor outcome following primary carpal tunnel release in non-diabetic patients. J Hand Surg Br 1994;19:622-625.


11. Hagberg M, NystrĂśm A, Zetterlund B: Recovery from symptoms after carpal tunnel syndrome surgery in males in relation to vibration exposure. J Hand Surg Am 1991;16:66-71.


12. Cagle PJ, Reams M, Agel J, Bohn D: An outcomes protocol for carpal tunnel release: a comparison of outcomes in patients with and without medical comorbidities. J Hand Surg Am 2014;39:2175-2180.


13. Mondelli M, Padua L, Reale F, Signorini AM, Romano C: Outcome of surgical release among diabetics with carpal tunnel syndrome. Arch Phys Med Rehabil 2004;85:7-13.

14. Jenkins PJ, Duckworth AD, Watts AC, McEachan JE: The outcome of carpal tunnel decompression in patients with diabetes mellitus. J Bone Joint Surg Br 2012;94:811-814.


15. Roh YH, Lee BK, Noh JH, Oh JH, Gong HS, Baek GH: Effects of metabolic syndrome on the outcome of carpal tunnel release: a matched case-control study. J Hand Surg Am 2015;40:1303-1309.


16. Zyluk A, Puchalski P: A comparison of outcomes of carpal tunnel release in diabetic and non-diabetic patients. J Hand Surg Eur Vol 2013;38:485-488.


17. Thomsen NO, Cederlund R, RosĂŠn I, BjĂśrk J, Dahlin LB: Clinical outcomes of surgical release among diabetic patients with carpal tunnel syndrome: prospective follow-up with matched controls. J Hand Surg Am 2009;34:1177-1787.


18. Thomsen NO, Cederlund RI, Andersson GS, RosĂŠn I, BjĂśrk J, Dahlin LB: Carpal tunnel release in patients with diabetes: a 5-year follow-up with matched controls. J Hand Surg Am 2014;39:713-720.


19. Porter P, Venkateswaran B, Stephenson H, Wray CC: The influence of age on outcome after operation for the carpal tunnel syndrome: a prospective study. J Bone Joint Surg Br 2002;84:688-691.

20. Lee J, Kwon YW, Choi JC, Choi JH, Lim HS, Kim SK: Prevalence of and risk factors for carpal tunnel syndrome in a rural population. J Korean Acad Rehab Med 2001;25:818-826.
21. Choi SJ, Ahn DS: Correlation of clinical history and electrodiagnostic abnormalities with outcome after surgery for carpal tunnel syndrome. Plast Reconstr Surg 1998;102:2374-2380.


22. Stone OD, Clement ND, Duckworth AD, Jenkins PJ, Annan JD, McEachan JE: Carpal tunnel decompression in the super-elderly: functional outcome and patient satisfaction are equal to those of their younger counterparts. Bone Joint J 2014;96-B:1234-1238.

23. Bodavula VK, Burke FD, Dubin NH, Bradley MJ, Wilgis EF: A prospective, longitudinal outcome study of patients with carpal tunnel surgery and the relationship of body mass index. Hand (N Y) 2007;2:27-33.



24. Higgs PE, Edwards DF, Martin DS, Weeks PM: Relation of preoperative nerve-conduction values to outcome in workers with surgically treated carpal tunnel syndrome. J Hand Surg Am 1997;22:216-221.


25. Nathan PA, Meadows KD, Keniston RC: Rehabilitation of carpal tunnel surgery patients using a short surgical incision and an early program of physical therapy. J Hand Surg Am 1993;18:1044-1050.


26. Kronlage SC, Menendez ME: The benefit of carpal tunnel release in patients with electrophysiologically moderate and severe disease. J Hand Surg Am 2015;40:438-444.


27. Jansen MC, Evers S, Slijper HP, de Haas KP, Smit X, Hovius SE, et al: Predicting clinical outcome after surgical treatment in patients with carpal tunnel syndrome. J Hand Surg Am 2018;43:1098-1106.


28. Katz JN, Losina E, Amick BC, Fossel AH, Bessette L, Keller RB: Predictors of outcomes of carpal tunnel release. Arthritis Rheum 2001;44:1184-1193.


29. Watchmaker JD, Watchmaker GP: Independent variables affecting outcome of carpal tunnel release surgery. Hand (N Y) 2017;13:285-291.


30. Ebrahimzadeh MH, Mashhadinejad H, Moradi A, Kachooei AR: Carpal tunnel release in diabetic and non-diabetic patients. Arch Bone Jt Surg 2013;1:23-27.


31. Fowler JR, Cipolli W, Hanson T: A comparison of three diagnostic tests for carpal tunnel syndrome using latent class analysis. J Bone Joint Surg Am 2015;97:1958-1961.


32. Deryani E, Aki S, Muslumanoglu L, Rozanes I: MR imaging and electrophysiological evaluation in carpal tunnel syndrome. Yonsei Med J 2003;44:27-32.


33. Bland JD: A neurophysiological grading scale for carpal tunnel syndrome. Muscle Nerve 2000;23:1280-1283.


34. Yang SN, Yoon JS, Kim SJ, Kim GS: A long-term follow up study of prognostic factors after carpal tunnel release. J Korean Acad Rehab Med 2006;30:580-583.
35. Schwartz MS, Chan TP: Carpal tunnel syndrome: age as an important factor. Muscle Nerve 1998;21:829-830.


36. Kim JY, Park HY, Kang SS: The importance of age as a factor of carpal tunnel syndrome management. Ann Clin Neurophysiol 2001;3:15-20.
37. Pham K, Gupta R: Understanding the mechanisms of entrapment neuropathies. Neurosurg Focus 2009;26:E7.

39. Dy CJ, Mackinnon SE: Ulnar neuropathy: evaluation and management. Curr Rev Musculoskelet Med 2016;9:178-184.



40. Fung BW, Tang CY, Fung BK: Does aging matter? The efficacy of carpal tunnel release in the elderly. Arch Plast Surg 2015;42:278-281.



41. Daniel D, Anthony AA, Machiel Z: Electrodiagnostic Medicine. 2nd ed. Philadelphia: Hanley & Belfus; 2002, p. 1068.
42. Kanatani T, Fujioka H, Kurosaka M, Nagura I, Sumi M: Delayed electrophysiological recovery after carpal tunnel release for advanced carpal tunnel syndrome: a two-year follow-up study. J Clin Neurophysiol 2013;30:95-97.








