전이성 뇌종양에서 확인된 말초운동신경의 연접횡단 변성: 2예에서 관찰된 전기생리학적 소견
Trans-Synaptic Degeneration of Peripheral Motor Axons from Metastatic Brain Tumors: Electrophysiological Findings in Two Cases
Article information
Trans Abstract
Denervation potentials obtained during electromyography (EMG) are usually considered as evidence of axonal degeneration in the peripheral nervous system (PNS). However, they may also appear when only central nervous system (CNS) lesions exist. Here, we present trans-synaptic degeneration from CNS lesions in 2 cases with electrophysiologic findings of motor axon degeneration caused not by PNS lesions, but by metastatic tumors involving the corresponding primary motor cortexes. When cancer patients show denervation potentials in needle EMG results, physicians usually consider PNS-related etiologies first, such as drug-induced generalized peripheral polyneuropathy or metastatic lesions (e.g., Pancoast tumor). However, metastatic brain tumors in the motor cortex could cause the same denervation potentials as PNS lesions, such as cervical polyradiculopathies or plexopathies. When PNS-type abnormalities are revealed on EMG in cancer patients, further screening to evaluate CNS lesions involving the corresponding corticospinal tracts should be included to ensure a timely and proper intervention.
Introduction
Cancer patients suffer from various neuromuscular complications during their clinical courses. If cancer patients mainly complain of lower motor neuron (LMN) symptoms, electrodiagnostic study (EDX) is usually recommended to localize any abnormalities in the peripheral nervous system (PNS).
Although abnormal spontaneous activities such as fibrillation and positive sharp waves are diagnostic characteristics of PNS pathologies, lesions of the central nervous system (CNS) generate such abnormalities in the needle electromyography (EMG) results through motor neuronal axonal degeneration [1,2]. This can cause confusion in interpreting electrophysiologic studies and diagnostic errors while planning appropriate therapy for cancer patients. Herein, we report 2 cases of motor weakness of LMN type with a huge amount of denervation potentials, actually caused by axonal degeneration from metastatic brain tumors.
Case Reports
Case 1
A 62-year-old female visited the outpatient clinic complaining of pain in her right shoulder and right upper extremity which lasted for 3 months. She underwent exploratory laparotomy and resection for ovarian cancer 2 years ago, followed by adjuvant chemotherapy with paclitaxel and carboplatin. Thereafter, approximately 1 year ago, a computed tomography (CT) scan further revealed newly appeared lymph node metastasis. During conservative care for recurred ovarian cancer, she suddenly experienced upper extremity pain and difficulty in moving her arm. On physical examination using the Medical Research Council (MRC) scale, the right shoulder abduction and elbow flexion were grade 3, while elbow extension, wrist dorsiflexion, wrist volar flexion, and finger flexion were grade 2. Muscle strength in the patient’s left arm was normal. Light touch sensation was diminished on her entire right arm and hand. The muscle stretch reflexes were increased in right biceps, brachioradialis, and bilateral quadriceps.
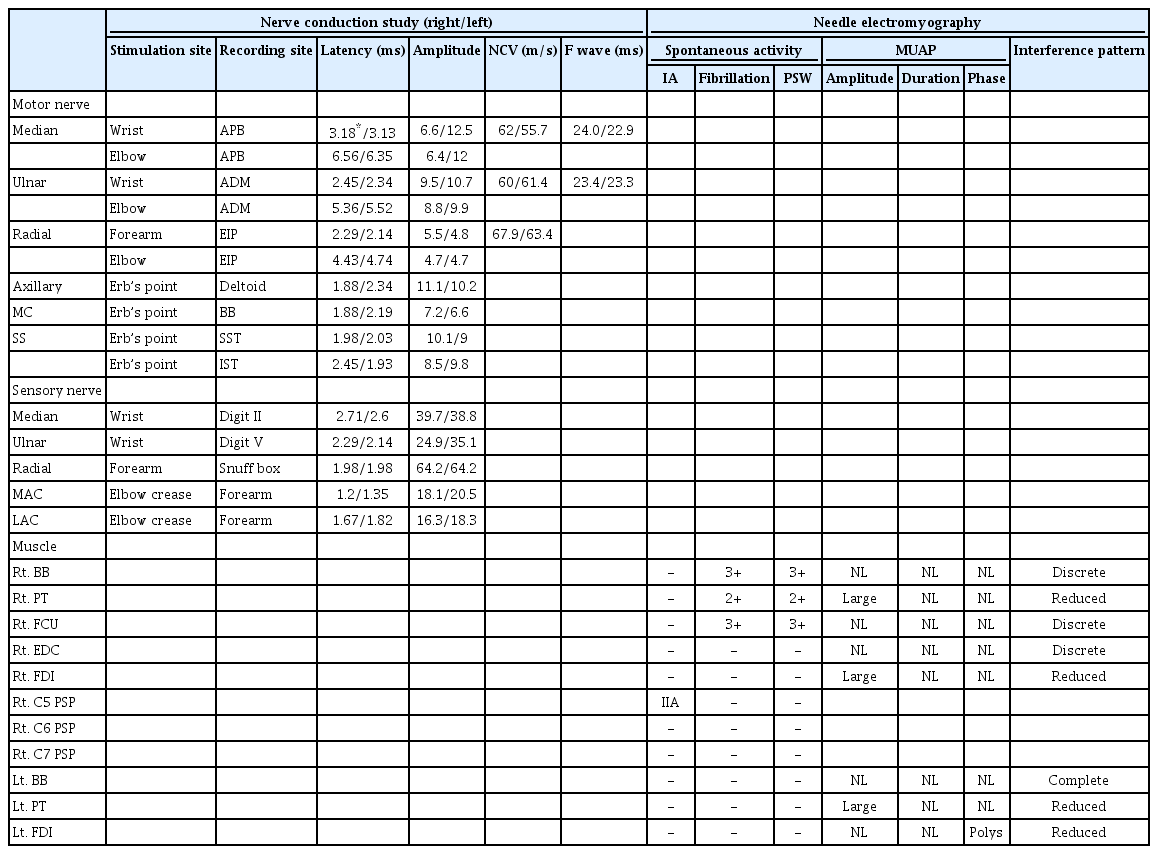
EDX was first performed to differentiate brachial plexopathy from a metastatic lesion versus cervical spinal metastasis, due to asymmetric, focal-distributed weakness. The nerve conduction study (NCS) results were normal. The needle EMG demonstrated profound fibrillation potentials and positive sharp waves in the right biceps, pronator teres, flexor carpi ulnaris muscles (Fig. 1A). The interference pattern was also reduced in the entire sampled muscles in her right upper extremity, and her left first dorsal interosseous and pronator teres muscle (Table 1).
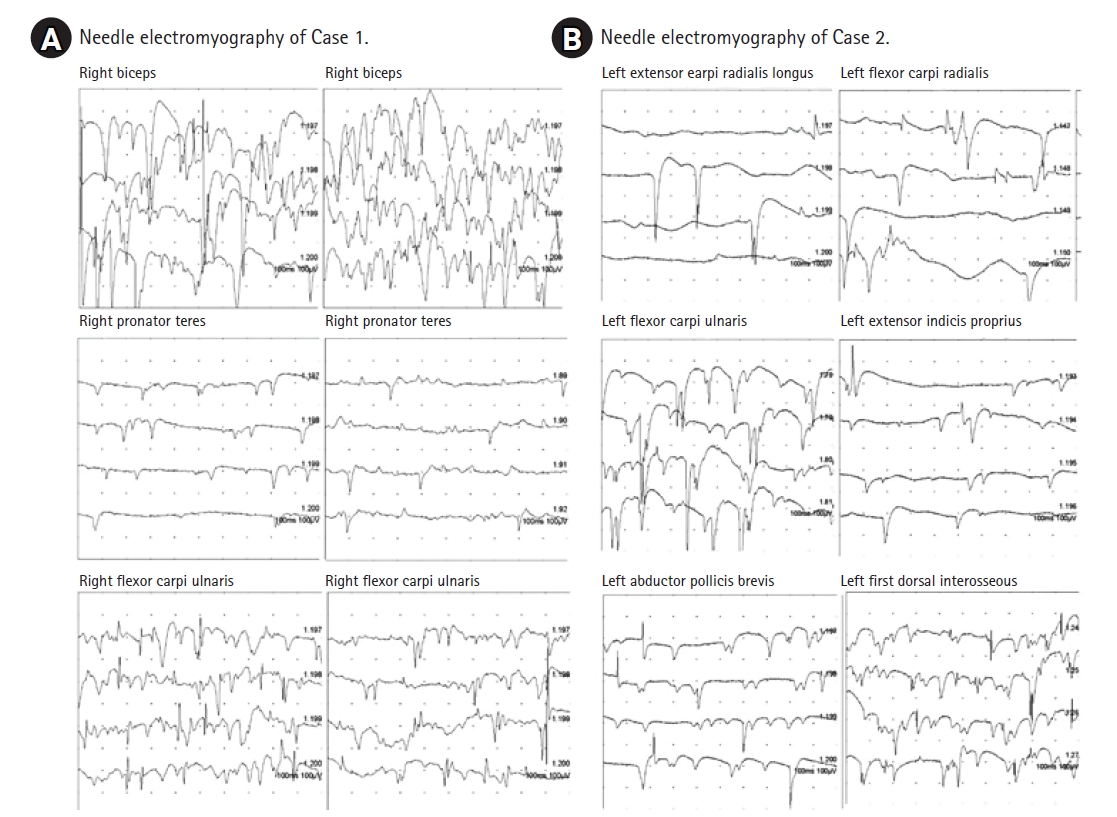
Electromyography shows abundant positive sharp waves and fibrillation potentials in the muscles of case 1 (A) and case 2 (B).
Based on the initial EDX indicating right cervical polyradiculopathies, we first suspected spinal metastasis as the primary cause for her pain and weakness. However, neither bone scan nor cervical spine magnetic resonance image (MRI) showed any evidence of bone or spinal cord metastasis. Subsequently, we conducted brain MRI which finally demonstrated multiple brain metastasis with perilesional edema in the left parietal lobe around the primary motor cortex, combined with lesions at the right temporal and the occipital lobes without involving the motor cortex (Fig. 2). She, therefore, underwent gamma knife surgery and chemotherapy for further management of brain metastasis.
Case 2
A 61-year-old male presented with left-side upper extremity weakness that had aggravated since the past month. He explained that initial weakness started from only hand but spread to his proximal arm. He had a history of lung cancer with lymph node metastasis (adenocarcinoma, stage IIIA) and underwent concurrent chemoradiotherapy a year ago. Previous CT and 18F-fluorodeoxyglucose positron emission tomography (PET) scans 6 months ago showed a reduced size of lung cancer and no more lymph node metastasis, suggesting a stable disease state. The C-spine MRI at the time of this newly aggravating weakness and pain showed no definite pathologies to cause any neurologic symptoms of his left arm.
On the physical examination, his left shoulder abduction, elbow flexion, and elbow extension were grade 3, and his left finger extension and finger abduction were grade 0 according to the MRC scale. He felt pain and tingling sensation on the medial side of his left forearm and hand. Muscle stretch reflexes were all normal. Atrophy was definite in the intrinsic muscles of his left hand. To differentiate left brachial plexopathy or chemotherapy-induced peripheral polyneuropathy, EDX was conducted 1 month after the onset of weakness. The NCS showed decreased medial antecubital cutaneous sensory nerve action potential on the left side, undetectable compound motor action potential on the left radial nerve, and prolonged latencies in bilateral median sensory and motor nerves. The NCS results in right upper limb were normal except median nerve (Table 2). We observed abundant abnormal spontaneous activities in the left abductor pollicis brevis, first dorsal interosseous, extensor indicis proprius, flexor carpi radialis, flexor carpi ulnaris, and extensor carpi radialis longus muscles (Fig. 1B). No motor unit action potential (MUAP) was noted in the left first dorsal interosseous, extensor indicis proprius, flexor carpi radialis, flexor carpi ulnaris muscles, and MUAP recruitment decreased in the left deltoid, supraspinatus, and latissimus dorsi muscles. Otherwise, cervical paraspinal muscles and muscles in the right upper limb were normal. The EDX result suggested possibilities of left brachial plexopathy mainly involving lower trunk level or post-ganglionic cervical polyradiculopathies.
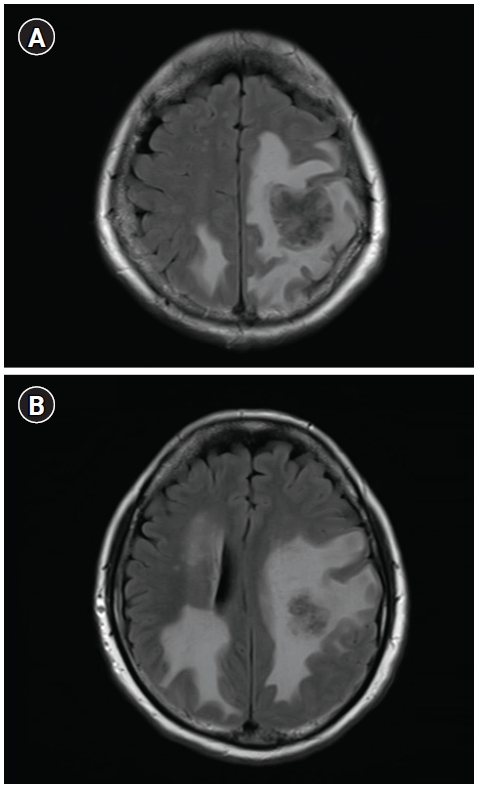
An immediate brain MRI followed when he visited the emergency room 15 days after the EDX due to abrupt progression of symptoms, weakness even in the lower limbs and gait disturbance. A cystic mass in the right frontoparietal lobe was diagnosed (Fig. 3). A PET scan showed the progression of lung cancer and hematogenous spread of multiple metastasis in the lung and brain, but without metastasis in the vertebrae, spinal cord, or brachial plexus. The patient was admitted and underwent gamma knife surgery for brain metastasis of lung cancer.
Discussion
Both patients developed LMN-type weakness and initially were misdiagnosed as radiculopathy or plexopathy according to the EDX. If cancer patient complains of neurologic symptoms, the following diagnoses should all be considered; direct effect of the primary tumor or metastasis involving the nervous system and the indirect effect of paraneoplastic syndrome, neural damage due to cancer treatment [3] or other idiopathic pathology such as neuralgic amyotrophy. In these cases, generalized peripheral polyneuropathy owing to paraneoplastic syndrome or chemotherapy was excluded based on the EDX abnormalities limited in unilateral arm. As the patient in case 1 complained prolonged weakness and pain more than 3 months and the patient in case 2 presented weakness without pain, the clinical course of two cases is different from that of neuralgic amyotrophy, typically presenting new-onset pain which develops suddenly and disappears and subsequent weakness [4]. Therefore, neuralgic amyotrophy could be ruled out. The possibility of lesions in anterior horn cell, spinal nerve root, or plexus, initially suggested following the EDX in our cases, was further excluded by additional spine MRIs or PETs. The brain metastasis damaging the corresponding motor cortex of symptomatic limbs, was only lesion that can explain patients’ weakness.
The EDX abnormalities in both patients, originated from the CNS lesions, can be explained by antegrade transneuronal degradation of α-motor neurons, also known as trans-synaptic degeneration (TSD) from motor cortex/corticospinal tracts. TSD is the spread of neuronal degradation into adjacent neurons caused by the loss of trophic signals of nearby neurons. Likewise, the deprivation of upper motor neuron control of α-motor neurons could further influence of axonal flow and result in axonal degeneration in the secondary motor neurons as well [2]. Alternatively, neurotoxic molecules or signals from metastatic lesions may transport from the upper motor neuron to the LMN to induce LMN degeneration.
This phenomenon was also previously described in patients with several other types of CNS lesions. Zhang et al. [5] demonstrated secondary neurodegeneration at the substantia nigra or ipsilateral thalamus after middle cerebral artery territory infarctions. Nonetheless, the existence of TSD through the corticospinal tracts outside the brain is still controversial. A postmortem study suggested that peripheral α-motor neurons were not likely to exhibit similar Wallerian degeneration of corticospinal tracts because other inputs from various afferent systems via interneurons continued [6]. However, an animal study discovered neuronal loss in the ventral horns in rats with infarction at the contralateral side [7], and severe secondary degeneration was also reported in peripheral motor axons below the level of injury in patients with spinal cord injury [8]. In stroke patients, denervation potentials were found at affected limbs after 2 to 3 weeks since onset, continued until 1 year, and usually diminished when volitional activity and spasticity developed. The abnormal spontaneous activities were more predominant in upper extremities than lower extremities, and in distal muscles than proximal ones, suggested as distal hand muscles receive more cortical innervations [2].
In addition to stroke, primary brain tumors also reported Wallerian degeneration in the corticospinal tracts [9,10]. However, the involvement pattern of TSD in cancer patients was not reported in previous studies. Although serial EDX were not followed, these two cases illustrated abundant denervation potentials 1 to 3 months after symptom, but no definite discrepancy between proximal and distal muscles. Further studies are required to evaluate the pattern, progression, and clinical impact of this TSD superimposed on already paretic limbs from CNS metastases. Since any CNS insult could cause an accompanying PNS dysfunction through this TSD, the functional disability of patients would be aggravated if this additional pathologies are ignored and only spontaneous recovery after CNS lesions are appreciated.
In conclusion, any metastatic CNS lesions involving corticospinal tracts responsible for weakened muscles should also be included in the list for differential diagnosis, even when cancer patients complained of focal, LMN-type neurologic symptoms. Although denervation potentials are usually considered as common evidence of PNS lesions, such fibrillations and positive sharp waves can appear from brain metastatic lesions if motor cortex fail to generate normal trans-synaptic signals to motor axons and peripheral muscles.
Notes
Conflict of Interest
No potential conflict of interest relevant to this article was reported.