|
 |
- Search
| J Electrodiagn Neuromuscul Dis > Volume 26(1); 2024 > Article |
|
Abstract
Acute dystonic reactions (ADRs) are movement abnormalities characterized by involuntary muscle contractions that typically manifest after exposure to a triggering agent, such as a medication. The specific muscle groups affected determine the type of reaction. For instance, an oculogyric crisis primarily affects the ocular muscles, while oromandibular dystonia involves jaw opening and tongue protrusion. We present the rare case of a 68-year-old man with amyotrophic lateral sclerosis who was successfully treated for an ADR. The patient was admitted with loss of consciousness due to respiratory failure. Tracheostomy was promptly performed under sedation with multiple general anesthetic agents. Immediately after tracheostomy, the patient communicated via eye-blinking without any notable abnormalities, just as before the procedure. However, the following day, he became unresponsive to verbal cues and exhibited a decreased level of consciousness, accompanied by tongue dyskinesia, deviation of both eyes to the left, and loss of visual tracking. The patient’s vital signs remained stable. Brain imaging and an electroencephalogram revealed no abnormalities. Treatment with midazolam produced initial improvement; however, due to a significant side effect of hypotension, the treatment was switched to oral diazepam. The patient’s condition gradually improved, and the medication was eventually discontinued without further ADR episodes.
Acute dystonic reactions (ADRs) are movement abnormalities characterized by involuntary muscle contractions that typically manifest after exposure to a triggering agent, such as a medication. The specific muscle groups affected determine the type of reaction. For example, an oculogyric crisis primarily affects the ocular muscles, leading to upward and outward deviations of the eyes, while oromandibular dystonia is characterized by jaw opening and tongue protrusion [1]. ADRs result from a dopaminergic-cholinergic imbalance in the basal ganglia [2]. Metoclopramide, a dopaminergic antagonist, is a common cause of ADRs [3]. Additionally, some case reports have indicated that propofol can induce ADRs, which in some instances may lead to life-threatening laryngeal spasms [4,5]. In this report, we present a case of an ADR accompanied by an oculogyric crisis that occurred following sedation with multiple general anesthetic agents for amyotrophic lateral sclerosis (ALS). The ADR was successfully managed with pharmacotherapy.
A 68-year-old man, diagnosed with ALS in 2020, was admitted to our hospital on March 7, 2023, due to a loss of consciousness. He exhibited respiratory failure that was attributed to the progression of ALS. Consequently, an arterial blood gas analysis was conducted, revealing a marked increase in carbon dioxide levels with a PCO2 of 90.8 mm Hg. Endotracheal intubation was promptly performed, followed by the initiation of mechanical ventilation to ensure adequate respiratory support. The following day, on March 8, the patient underwent bedside tracheostomy while under general anesthesia in the intensive care unit (ICU).
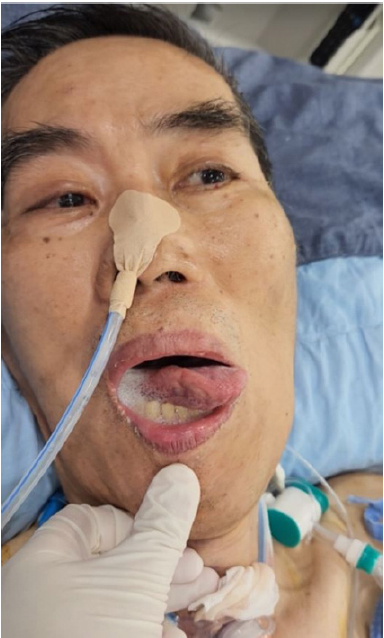
Until 1:20 PM on March 9, the patient was still able to communicate by blinking his eyes, with no notable cognitive impairment, and his muscle strength remained at grade zero, consistent with his condition before tracheostomy. However, at approximately 1:30 PM, he became unresponsive to verbal cues and experienced a decrease in consciousness. This was accompanied by tongue dyskinesia and deviation of both eyes to the left, as well as a loss of visual tracking (Fig. 1, Supplementary Video 1). The patient’s vital signs remained stable. Computed tomography and diffusion-weighted magnetic resonance imaging of the brain were performed to determine the underlying cause, but neither revealed any evidence of brain lesions, including acute stroke. An electroencephalogram also did not show any evidence of seizure activity. To our knowledge, the literature includes no reported cases of these symptoms appearing as a result of ALS progression.
Considering the patient’s symptoms and circumstances at the time, the most plausible explanation is that an ADR occurred, manifesting as an oculogyric crisis following the administration of sedative medications (fentanyl and propofol).
On the first day of symptom onset, a 3-mg intravenous bolus of midazolam, a benzodiazepine, was administered with the goal of controlling the patient’s symptoms. Thirty minutes after administration, the patient displayed an improvement in eye closure and sedation, as well as a positive response in tongue movement. One hour after receiving midazolam, the patient regained awareness and responded appropriately to one-step verbal commands. However, after 4 hours, he experienced a recurrence of impaired consciousness and tongue dyskinesia. This led to considerations regarding the pharmacokinetics of midazolam, which has a half-life of approximately 3 hours before its effectiveness significantly decreases. Due to its strong hypotensive effect, the repeated use of midazolam was limited. On the second day, oral administration of diazepam was initiated at a dose of 2 mg three times per day, while the administration of midazolam was discontinued. However, no notable improvements were observed in the patient’s symptoms that day. On the third day, the patient consistently exhibited an alert mental state and responded appropriately to one-step verbal commands. Although limitations in smooth conversation persisted due to difficulties vocalizing after tracheostomy, the patient demonstrated appropriate responses to oral commands, including instructions to close his eyes and blink twice. His tongue dyskinesia was also reduced (Fig. 2, Supplementary Video 2). The patient’s cognitive function remained unchanged from before the tracheostomy procedure, and the muscle strength in his limbs continued to receive a grade of zero. On day 15 after symptom onset, as the patient’s symptoms gradually improved, the administration of diazepam was discontinued. No further ADR episodes or oculogyric crises occurred after the medication was stopped.
Written informed consent was obtained from the patient and his guardian.
ADRs are known to occur due to cholinergic hyperactivity and dopaminergic hypoactivity in the nigrostriatal pathway and are commonly induced by dopamine-blocking agents, such as both typical and atypical antipsychotics or antiemetics [2,3]. Reports have also described ADRs induced by anesthetic agents [4,5]. In the case presented here, mechanical ventilation was initiated 1 day before the onset of the patient’s symptoms, with continuous use of fentanyl and dexmedetomidine for sedation. Therefore, 12 hours prior to the onset of symptoms, the patient had been administered fentanyl, dexmedetomidine, and propofol in connection with tracheostomy. While propofol has been widely reported as an anesthetic agent that can cause ADRs when used alone, the literature includes few reports of ADRs associated with the combination of fentanyl and dexmedetomidine, as our patient received [6]. The mechanism behind ADRs caused by these anesthetic agents may involve an imbalance between dopaminergic and cholinergic neurotransmission in the basal ganglia circuit, since a fine balance between dopamine and acetylcholine receptors is essential for neuromuscular coordination [4]. ADRs can manifest with a variety of clinical symptoms, including oculogyric crisis, oromandibular dystonia, and laryngeal dystonia [1,2]. Although laryngeal dystonia is only rarely reported to be caused by neuroleptics, it is potentially life-threatening [7,8]. Notably, dystonic reactions in the airways can be fatal in cases of neuromuscular disease. Consequently, the early diagnosis and treatment of ADRs are of paramount importance.
Treatment options for ADRs include the intravenous administration of benzodiazepines, such as diazepam and midazolam, or anticholinergic agents (e.g., benztropine). Antihistaminergic agents may also be considered in some cases [1,2]. In our case, we initially administered midazolam as the first-line of treatment and observed improvements in the patient’s oculogyric crisis and tongue dyskinesia 30 minutes after administration. However, symptoms recurred 4 hours later, likely due to the diminishing effects of the drug after its 3-hour half-life. Nevertheless, the continued use of midazolam should be approached with caution due to its potential hypotensive effects.
In Thailand, a patient with severe myasthenia gravis experienced a reaction following the administration of multiple anesthetic agents and received treatment with intravenous diazepam [6]. In our case, diazepam was given orally, considering its hypotensive effects, and the patient’s dystonic reactions quickly subsided. We attribute this outcome to diazepam’s inhibition of the tonic release of dopamine, which in turn influences the balance between dopaminergic and cholinergic activity [9].
Importantly, patient conditions sometimes deteriorate, necessitating transfer to the ICU and the initiation of mechanical ventilation. Consequently, prompt implementation of brain imaging and electroencephalography is crucial for differential diagnosis, followed by the application of appropriate conservative measures and careful monitoring. In certain circumstances, immediate brain imaging may be difficult due to the patient’s condition. It is advantageous to quickly determine whether clinical symptoms suggestive of ADR manifest after the initial administration of a sedative drug, even when brain imaging cannot be performed right away. In such instances, the intravenous administration of benzodiazepines may represent a suitable first-line option for symptom management. It is imperative to recognize that ADRs can result from the use of general anesthetic agents and may pose a threat to the patient’s life. Therefore, prompt medical attention in an emergency department is strongly advised.
Acknowledgments
This work was supported by a clinical research grant from Pusan National University Hospital in 2023.
Supplementary materials
Further details on supplementary materials are presented online (available at https://doi.org/10.18214/jend.2023.00157).
References
2. Lewis K, O'Day CS: Dystonic reactions. In: StatPearls. Treasure Island: StatPearls Publishing; 2022 [cited 2024 Apr 11]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK531466
3. Dingli K, Morgan R, Leen C: Acute dystonic reaction caused by metoclopramide, versus tetanus. BMJ 2007;334:899-900.



4. Schramm BM, Orser BA: Dystonic reaction to propofol attenuated by benztropine (cogentin). Anesth Analg 2002;94:1237-1240.


5. Steckelberg RC, Tsiang D, Pettijohn K, Mendelsohn A, Hoftman N: Acute vocal fold dystonic reaction to propofol: a case report. Am J Otolaryngol 2015;36:303-305.


6. Jitprapaikulsan J, Srivanitchapoom P: Acute dystonic reaction following general anesthetic agent use. Tremor Other Hyperkinet Mov (N Y) 2017;7:514.



7. Koek RJ, Pi EH: Acute laryngeal dystonic reactions to neuroleptics. Psychosomatics 1989;30:359-364.


8. O’Neill JR, Stephenson C: Antipsychotic-induced laryngeal dystonia. Psychopharmacol Bull 2022;52:61-67.