 |
 |
- Search
| J Electrodiagn Neuromuscul Dis > Volume 24(1); 2022 > Article |
|
Abstract
Facial nerve palsy (FNP) can be a challenging medical condition, and bilateral FNP is an uncommon occurrence that is potentially fatal and warrants urgent medical intervention. We report a rare case of bilateral FNP that developed after traumatic brain injury (TBI), which we approached through an electromyographic study. A 23-year-old male patient had experienced a fall-down injury from height of 4 meters while on his military service. Computed tomography of the brain and facial bone showed acute TBI and multiple skull base fractures. Limited facial expression and dysarthria started at the time of cranioplasty, which was about 3 months after the patientŌĆÖs initial presentation, and these symptoms gradually deteriorated over time. An electrodiagnostic study demonstrated incomplete bilateral facial nerve lesions, which were strongly indicated as lower motor neuron lesions. An early diagnosis based on electrodiagnostic study should be considered for proper treatment, which can achieve optimal functional recovery after bilateral FNP.
Facial nerve palsy (FNP) can be a challenging medical condition, and patients with facial nerve disorders often faces various psychological obstacles [1]. Wide variety of etiologic factors exists causing facial paralysis, including Bell palsy, underlying medical conditions such as Guillain-Barre syndrome, leukemia, or infectious mononucleosis, and trauma [2]. Unilateral FNP is relatively common, reporting incidence of 20 to 25 per 100,000 population, whereas bilateral FNP is a rare clinical occurrence, which accounts for approximately 0.3 to 2 percent in facial palsy cases [2,3]. Bilateral FNP is usually found in systemic medical conditions, which are often potentially fatal and frequently warrant urgent medical intervention [4]. Among these causes, trauma is an extremely rare entity of bilateral facial paralysis [5].
We report an uncommon case of bilateral FNP proposed after traumatic brain injury (TBI), using electromyographic study to approach this medical condition.ŌĆā
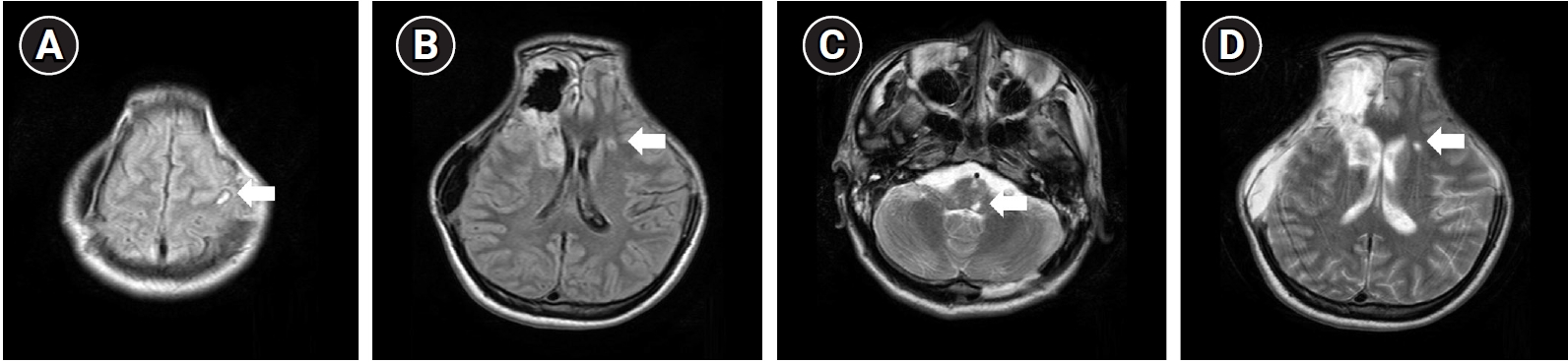
A 23-year-old male patient who had no medical comorbidity had experienced a fall down injury from height of 4 meters while on his military service. On the initial neurological examination, his mental status was stuporous, and he had been admitted to the emergency department. According to the medical record, there was no skin laceration or muscle injury in the face other than head trauma, and no treatment history involved as well. Initial Glasgow Coma Scale was rated for 9, eye response for 3, verbal response for 2, and motor response for 4, respectively. Computed tomography (CT) of brain and cranium showed acute subarachnoid hemorrhage and acute epidural hemorrhage on right frontotemporal lobe and left occipital lobe, accompanied with multiple skull base fractures in both sides (Fig. 1). No other facial bone fractures were seen in the initial CT. Magnetic resonance imaging (MRI) was taken after 1 month from initial injury, which showed multiple T2 hyperintensities in left cerebral hemisphere and brainstem, suggesting diffuse axonal injury (Fig. 2). He had been transferred to the department of neurosurgery, and decompressive craniectomy and hematoma removal had been done. After about 2 months later, cranioplasty was conducted. He has received comprehensive rehabilitation therapy including physiotherapy, occupational therapy, and pulmonary rehabilitation throughout several hospitals. After 2 years from initial onset, he was transferred to our hospital to receive continuous rehabilitation therapy.
On admission day of our hospital, he showed limited facial expression and often spilled food residue on both side of while eating. Symptoms had been developed after cranioplasty, which was about 3 months later from initial onset, and gradually got worsened over time. Furthermore, owing to his severe dysarthria, it was often difficult to understand even at the word level without contextual clues, and controlling articulation in all consonants was incomplete. Videofluoroscopic swallowing study revealed incomplete laryngeal closure and elevation, as well as cricopharyngeal dysfunction. He showed obvious bilateral facial weakness with disfiguring asymmetry, which was more prominent on left side, evaluated as grade IV for left side and grade II for right side, according to House-Brackmann (H-B) grading system. Specifically, there was no movement for wrinkling forehead, incomplete eye closure and asymmetry for grinning mouth with maximal effort for left side, and slight weakness for wrinkling forehead and mouth movement, and complete eye closure with minimal effort for right side. There was no facial sensory change or hearing loss on both sides. Prior to our hospital, diagnostic approaches associated with these symptoms have never been conducted.
We performed electrodiagnostic approach for his bulbar symptoms, which can discriminate the etiology of bilateral facial palsy. According to the result, facial motor nerve conduction study stimulating bilateral facial nerves at preauricular area showed prolonged onset latency in nasalis branches, and low amplitude of combined motor action potential in frontalis, oculi, nasalis, and oris branches of bilateral facial nerves (Table 1). Needle electromyography showed abnormal spontaneous activities in the bilateral orbicularis oculi and orbicularis oris muscles, and it also showed decreased interference patterns at all examined muscles (Table 2). Blink reflex study stimulating both supraorbital nerves showed no responses for both R1, ipsilateral R2 and contralateral R2 (Table 3). These results corresponded with incomplete bilateral facial nerve lesions, which highly indicates lower motor neuron lesions. Despite consecutive rehabilitation program including speech therapy, occupation therapy and transcutaneous electrical stimulation therapy, severity of FNP showed no interval change in a 6-month follow-up at outpatient basis.
The patient was informed that data concerning the cases would be submitted, and he provided informed written consent for publication of this case report and accompanying images.
Post-traumatic FNP usually results from fracture of the base of the skull or acoustic trauma [5]. To our knowledge, the present case is unique in that, delayed onset and persistent bulbar symptoms appeared after TBI were diagnosed as bilateral FNP via electrodiagnostic approach. Compared with the previously reported cases of bilateral FNP after trauma [6-8], there is a difference between the timing of symptom onset and electrodiagnostic study. One of the primary causes of bulbar symptoms such as decreased facial expression and dysarthria after cranioplasty in our patient was bilateral temporal bone fracture, which was initially confirmed by CT. Delayed onset of facial paralysis may be the result of bleeding into the surrounding structures of facial nerve, leading to gradual edematous status which can induce external compression of the nerve [8]. Moreover, existence of blood clots in an artery or vein can lead to ischemic damage and a compressive effect [8]. Considering the gradual deterioration after cranioplasty, the possibility of facial nerve damage during surgery cannot be excluded.
Electrodiagnostic approaches for bilateral FNP are performed through facial nerve conduction and blink reflex study. No responses for blink reflex study can be observed as in our case, where the severity of the damage and the prolonged symptom onset may have affected the test results. Additional evaluation such as brain imaging might be required depending on the results. Furthermore, it is also important in determining whether to perform treatment targeting peripheral nerve lesions regarding the rehabilitation strategies, such as electrical stimulation therapy. Obtaining degeneration ratio through electroneurography is significant to predict recovery from facial palsy. It was suggested that sequelae of facial palsy might persist, and effect of the treatment might not be favorable for our patient. Additional tests such as trigeminal somatosensory evoked potential might have been required, which was unfeasible due to poor cooperation of the patient.
When bulbar symptoms affecting quality of life emerge from the patient, an early electrodiagnostic study can be performed for the prompt initiation of rehabilitation treatment through early diagnosis in the patient with bilateral FNP. Physicians should get acquainted that delayed onset bulbar symptoms may occur in patients with TBI, despite the absence of bilateral temporal bone fractures, and symptoms may not appear in the early stages. Outcomes of TBI are often fatal especially when parenchymal injury is present, which leads to failure of early detection of bilateral FNP. Early diagnostic intervention may be necessary if patients exhibit incomplete eye closure or labile affect at the time of admission. Since an early electrodiagnostic study can be significant evidence to determine the possibility of surgery, being alert to these symptoms might be the attitude that physicians dealing with TBI patients should have.
Our case has a limitation regarding the measurement tool. We used H-B scale for evaluation tool, and since it is a scoring system that comprehensively grades each section of the face, overall scoring may be inaccurate if a large deviation exists for each section. Furthermore, bilateral FNP would not be easily recognizable in H-B scale due to difficulty of comparing both sides. In our case, however, left side showed more severe feature compared to right side, which might have been acceptable for applying H-B scale.
In conclusion, our case presents rare medical condition of delayed onset bilateral FNP occurred after TBI, which can be a diagnostic challenge. Early diagnosis based on electrodiagnostic study should be considered for proper treatment, which can achieve optimal functional recovery after bilateral FNP.
Fig.┬Ā1.
Initial brain computed tomography (CT) of the patient. Axial view of brain CT (A-C) showed traumatic epidural hemorrhage in the right frontotemporal lobe and left occipital lobe, and acute subarachnoid hemorrhage. Coronal view of facial bone CT (D, E) demonstrated multiple skull base fractures on both sides (white arrows).
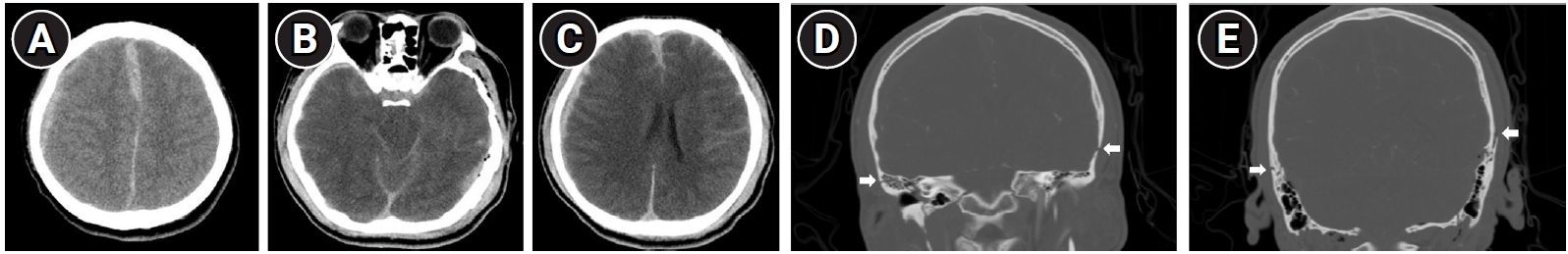
Fig.┬Ā2.
Brain magnetic resonance imaging of the patient taken 1 month after the initial injury. T2-weighted images including fluid-attenuated inversion recovery (A, B) and turbo spin echo (C, D) sequences showed hyperintensities in the left cerebral hemisphere and brainstem, indicating lesions of subcortical white matter, which demonstrated diffuse axonal injury (white arrows).
Table┬Ā1.
Facial Motor Nerve Conduction Studies
Table┬Ā2.
Needle Electromyography
References
1. Habib SS, Al Rouq F, Meo I: Post-Traumatic Bilateral Facial Paralysis Associated with Temporal Bone Fracture. J Coll Physicians Surg Pak 2015;25 Suppl 2:S132-3.

2. Gaudin RA, Jowett N, Banks CA, Knox CJ, Hadlock TA: Bilateral Facial Paralysis: A 13-Year Experience. Plast Reconstr Surg 2016;138:879-87.


3. ┼×ahin C, ├¢zen ├¢: Bilateral Post-Traumatic Facial Paralysis That Contains Longitudinal and Transverse Temporal Fracture. J Craniofac Surg 2018;29:1305-6.


5. Chitkara N, Bakshi N, Goyal N, Goyal P: Post-traumatic bilateral facial nerve palsy. J Otolaryngol 2002;31:192-3.


6. Abrah├Żo NM, Guimar├Żes GC, Castilho AM, da Silva VAR: Bilateral facial paralysis secondary to temporal bone trauma: A case report and literature review. Clin Case Rep 2021;9:e04272.



-
METRICS

-
- 0 Crossref
- Scopus
- 1,709 View
- 23 Download
- Related articles in J Korean Assoc EMG Electrodiagn Med
-
Unilateral Phrenic Nerve Palsy Following Blunt Chest Trauma: A Case Report2021 December;23(3)






