 |
 |
- Search
| J Electrodiagn Neuromuscul Dis > Volume 24(2); 2022 > Article |
|
Abstract
Objective
This study investigated the correlations between magnetic resonance imaging (MRI) and nerve conduction studies (NCS) in patients with ulnar neuropathy at the elbow (UNE).
Methods
In total, 46 patients who underwent elbow MRI and NCS at a single center from 2014 to 2018 were included. Motor studies, including segmental and inching tests, and sensory NCS were performed. The 5-point severity score was evaluated based on the signal change and swelling in the fat-suppressed T2 weighted sequence. The findings of MRI and NCS were grouped into 3 categories. The Spearman rank test was used to evaluate correlations between the severity score on MRI and electrodiagnostic parameters.
Results
The locations of the lesions on MRI and NCS were correlated in 20 of the 46 patients with UNE, while the other 20 patients had no correlations. Six patients who could not be categorized according to the location showed various findings. The severity score based on MRI showed significant negative correlations with amplitude on the segmental study (r = -0.423, p = 0.002) and the inching study (r = -0.456, p = 0.002), and with conduction velocity in the segmental study (r = -0.526, p < 0.001) and the inching study (r = -0.548, p < 0.001).
Ulnar neuropathy at the elbow (UNE) is the second most common mononeuropathy after carpal tunnel syndrome [1]. Neurophysiologic studies, including motor nerve conduction studies (NCS) and needle examinations, are commonly used to confirm the diagnosis of UNE. Although short segmental incremental studies have played a role in the localization of demyelinating UNE [2,3], this method of diagnosing UNE has several limitations. First, localization is not easy in some patients with mild lesions or in those who have severe axonal injuries with low distal compound muscle action potential (CMAP) amplitudes [1,4]. Second, there can be errors in distance and latency measurements [3,4]. Third, the sensitivity and specificity of this method are not very high [2,4].
Ultrasonography can also be used for the diagnosis of ulnar neuropathy, but its sensitivity and specificity are likewise lower than ideal [5,6]. Magnetic resonance imaging (MRI) has become increasingly important for the evaluation of UNE. Previous studies evaluating the diagnostic value of MRI findings of hyperintensity and nerve swelling showed varying results for sensitivity and specificity [5,7].
Although there have been many studies on UNE, few have investigated the relationship between MRI and electrodiagnostic studies in patients with UNE using segmental and inching studies to localize the location and determine its severity. The aim of this study was to investigate the correlations between MRI and NCS, especially segmental and inching studies, in patients with UNE.
Electronic medical records were searched for 194 patients diagnosed with or suspected of having UNE through electrodiagnosis from 2014 to 2018. In total, 46 patients who underwent elbow MRI and NCS were included in this retrospective study conducted at a single center. Patients who did not have a formal MRI reading or for whom the location of the lesion was difficult to identify through electrodiagnosis were excluded from the study. This study was exempt from Asan Medical Center Institutional Review Board review (No. 2022-0619).
The location of the lesion, based on both MRI and NCS, was divided into 3 sections. Section 1 included the area from 2 cm above the elbow to 6 cm above the elbow, section 2 included the area from 2 cm above the elbow to 2 cm below the elbow, and section 3 included the area from 2 cm below the elbow to 6 cm below the elbow. The location was categorized as “undetermined” if there was a broad lesion or if there were multiple lesions across the sections. A cross-sectional study design was used to evaluate the correlations of the localizations.
Neurophysiological testing was performed using an Oxford Synergy apparatus (Oxford Instruments; Medelec, Surrey, United Kingdom). Motor and sensory NCS were performed by electrical stimulation of the bilateral ulnar nerves, with the elbow flexed at 90°. For the motor NCS, CMAPs were recorded from the abductor digiti minimi muscle with surface electrodes, with filter settings of 3 Hz to 10 kHz, sensitivity of 5 mV for amplitude and 500 mV for onset latency determination, and sweep of 20 ms.
With clinical suspicion of UNE, both segmental and inching studies were conducted on the symptomatic side, and routine conduction studies were conducted on the wrist and the ulnar groove of the asymptomatic side [3]. In the segmental studies, 3 points (the wrist, below the elbow, and above the elbow) were stimulated. The diagnosis of UNE was based on electrophysiological criteria [3,8]. In the inching studies, 5 points of the ulnar nerve were stimulated at successive 2-cm intervals from 4 cm below to 6 cm above the medial epicondyle. Latency of more than 0.4 ms was as a positive finding [9]. The inching study was analyzed because it had shorter segments than the segmental study, leading to the possibility that it could yield more significant values for certain parameters, such as the conduction velocity.
Sensory nerve action potentials (SNAPs) were obtained antidromically from the fifth finger, with filter settings of 20 Hz to 2 kHz, sensitivity of 20 μV, and sweep of 10 ms. The needle examination included the first dorsal interosseous muscle and other ulnar innervated forearm flexors.
T2-weighted fat-saturated axial images focused on the radio-humeral joint were analyzed by 2 musculoskeletal radiologists, who reached consensus regarding their interpretation. The severity of each lesion was determined based on increased T2 signal intensity and nerve swelling in a cross-sectional image of the ulnar nerve through a qualitative image analysis. T2 signal intensity was qualitatively analyzed throughout the entire image. T2-weighted images without any hypersignal changes were considered “normal,” while those with a hypersignal change in the ulnar nerve were considered “abnormal” and further evaluated in terms of whether the change in the signal intensity was mild or severe. Mild swelling was defined as a more than 20% increase of the nerve caliber in relation to the proximal and distal segments, while severe swelling was defined as a more than 50% increase in the nerve caliber. The severity scoring for each section was conducted by 2 radiologists using a 5-point score from 0 to 4, which was based on the signal alteration and/or swelling on the fat-suppressed T2 weighted axial images [1,10]. Fig. 1 shows representative image sections.
Statistical analyses were performed using IBM SPSS ver. 25.0 (IBM Corp., Armonk, NY, USA). The Spearman correlation method was used to evaluate the correlation between the severity score on MRI and each electrodiagnostic parameter. Values of p<0.05 were considered to indicate statistical significance.
We reviewed 46 patients (28 males and 18 females) with available elbow MRI and NCS findings. The clinical and electrophysiologic findings of the 46 patients are described in Table 1. The mean age of the patients was 45.1 ± 16.9 years. On the day of the electrophysiologic study, 29 patients (63.0%) complained of sensory symptoms and signs without muscle weakness. Five patients (10.9%) had weakness of the ulnar-innervated muscles without sensory symptoms and signs. Twelve (26.1%) patients reported both sensory symptoms and muscle weakness. All studies were consistent with UNE. There were no absent findings of CMAP, and the ulnar CMAP was reduced in 12 patients (26.1%). Meanwhile, the ulnar SNAP was absent in 9 patients (19.6%) and reduced in 14 patients (30.4%). The absolute motor nerve conduction velocity from above-elbow to below-elbow was less than 50 m/s in 38 patients (82.6%).
The MRI findings of 46 patients revealed ulnar nerve abnormalities at the elbow in 43 (93.5%) patients. Eighteen patients had isolated ulnar nerve hyperintensity, including 15 patients with a mild T2 signal change (score 1) and 3 patients with a severe T2 signal change (score 2). A combination of ulnar nerve hyperintensity and mild swelling of the ulnar nerve at the elbow was the most common abnormality, seen in 18 patients (score 3), while 7 patients (score 4) had both signal changes and severe swelling of the ulnar nerve.
Table 2 shows the relationship of the lesion localization between MRI and the electrodiagnostic study for 46 patients. Six patients were classified as having an “undetermined” location based on the MRI findings, while 20 patients showed correlated locations between the MRI and electrodiagnostic studies. Three patients had normal MRI and abnormal electrodiagnostic findings, and 4 patients had normal electrodiagnostic and abnormal MRI findings.
Table 3 and Fig. 2 show some correlations between the severity score on MRI and the parameters on the segmental and inching studies. The amplitude of sensory NCS showed a negative correlation with the MRI-based severity score (r=-0.421, p=0.004). In the segmental studies, the severity score based on MRI was correlated with the amplitude and conduction velocity in the same section of the lesion. In the inching studies, the severity score based on MRI was correlated with the amplitude and the conduction velocity in the matched section of the lesion. The severity score based on MRI was also correlated with the minimum values of the amplitude and the conduction velocity among the 5 points that we stimulated.
This is the first study to reveal a correlation between MRI and NCS findings, including segmental and inching studies, in an investigation of the location and the severity of UNE. Most cases of UNE occur at the level of the retroepicondylar groove, which is called the cubital tunnel. This nerve may also be compressed at the humeral-ulnar aponeurosis between the 2 heads of the flexor carpi ulnaris (FCU) or at the point of the exit from the FCU [11]. Identifying the location of the entrapment helps with therapeutic decisions, because operative interventions such as resection of the arcuate ligament, medial epicondylectomy, and anterior transposition of the ulnar nerve can resolve cubital tunnel syndrome in some cases [12].
The ulnar nerve signal on T2 imaging has diagnostic importance in lesion localization since it is correlated with the segmental study of NCS. Increased signal intensity on T2 imaging indicates the presence of UNE, and the magnitude of nerve caliber enlargement distinguishes between mild and severe neuropathy [10]. Our study included both segmental and inching studies to determine the correlation between MRI and NCS results of UNE.
A previous study showed that ultrasonographic abnormalities due to UNE might not be maximal at the site of the nerve dysfunction based on the segmental and inching studies [13]. However, our study showed a correlation of severity between MRI and NCS based on the segmental and inching studies, without a significant difference between the segmental and inching studies..
The findings of high signal intensity and nerve swelling on MRI represent focal demyelination. Previous studies reported that MRI was more sensitive than segmental NCS studies, especially in patients with non-localizing UNE. A previous study found no significant differences in MRI findings according to different scores of UNE severity [1]. However, our study confirmed that MRI and NCS produced similar findings and that the UNE severity based on the NCS was correlated with the MRI findings, as in the 25 patients who showed abnormal spontaneous activity on needle EMG.
Furthermore, in a comparison between 2 subgroups, this study also revealed that more significant signal changes on elbow MRI were observed in patients with non-traumatic etiologies. Subgroup A included 18 patients with trauma and 3 patients with ulnar nerve subluxation. All 3 patients with ulnar nerve subluxation and 6 patients with traumatic history showed mild signal changes, with an MRI severity score of 1. Two patients with normal findings on MRI were confirmed to have UNE based on NCS. More than half of the patients with an MRI severity score of 3 and all patients with a score of 4 belonged to subgroup B, without traumatic etiology or subluxation. In particular, all patients with a severity grade of 4 showed severe osteophytes and bony spurs on elbow MRI. Therefore, it is inferred that MRI signal changes are more closely correlated with non-traumatic etiologies.
Among 20 patients with discrepancies in the location between the NCS and MRI, 9 had a previous history of orthopedic surgery. Among these patients, 6 had MRI findings superior to those of NCS, while 3 patients had NCS findings superior to those of MRI. Among 6 patients in the undetermined group based on MRI imaging, 5 patients had undergone previous orthopedic surgery and showed multiple lesions across the sections. Although there was a clinical correlation between the location of the lesion on MRI and NCS in 50% of the patients, the other patients had discrepancies that may have been related to the differences in the patient's position during NCS and MRI. A moderately flexed position (about 70° to 90°) has been recommended to ensure that surface skin measurements more closely reflect the true nerve length. An extended elbow position often causes an underestimation of the true nerve length, leading to artifactual delayed conduction velocity across the elbow. However, MRI is usually performed in the elbow-extended position [9,14,15]. Furthermore, another limitation is that the interval of the sections on MRI and the inching study were not exactly matched.
We did not conduct any further analyses of the SNAP amplitude because it is a limited parameter that depends on distance. In addition, the onset or peak latencies of SNAP could not be analyzed for quantitative correlations with the severity score based on the MRI findings because there were several patients with no SNAP responses. Although a previous study reported abnormalities on sensory NCS with normal findings on MRI, abnormalities on sensory NCS usually suggest the possibility of axonal degeneration [16]. This study showed a negative correlation between SNAP and MRI findings. To be more concrete, 4 patients had a score of 4 on MRI with hyperintensity and severe swelling of the ulnar nerve, and 3 of the 9 patients with absent SNAP findings had the lowest score on MRI, with mild hyperintensity.
This study has several limitations. First, the relatively small sample size made it difficult to determine the correlation of the location of the lesion based on NCS and MRI with the operative findings. Although the medical records of 194 patients with NCS were reviewed, only 46 patients underwent MRI for ulnar neuropathy, and only 14 patients had surgical records that confirmed the exact location of the lesion. Additional studies with larger sample sizes would reveal more definite correlations. Second, unblinded radiologists evaluated the MRI and qualitatively scored the severity of ulnar nerve entrapment. It would be preferable to correlate the severity of NCS and the quantitative scoring of MRI after measuring the interrater and intrarater reliabilities. Furthermore, it was not feasible to determine the clinical relationship of patients’ subjective symptoms with the severity and the location of the lesion based on NCS and MRI due to the insufficient symptomatic data in the medical records. Additional prospective studies including precise information on the distribution and the severity scales of the symptoms would give more useful information for the diagnosis of UNE.
Although some discrepancies about the location of the UNE existed between the electrodiagnostic studies and MRI, this study demonstrates that the electrodiagnostic outcomes have correlations with the severity score, which reflects the structural changes on MRI. In conclusion, using a combination of electrodiagnostic studies and MRI improves diagnostic accuracy, including the lesion localization and assessment of the severity of UNE. It is necessary to verify the relevance and localization of patients’ symptoms through additional prospective studies.
Fig. 1.
The severity based on the magnetic resonance imaging (MRI) findings using a 5-point score was evaluated based on the signal alteration and/or swelling on the fat-suppressed T2 weighted axial sequences. The images around the elbow were divided into 3 sections from 2 to 6 cm above the medial epicondyle (section 1), from 2 cm below the medial epicondyle to 2 cm above the medial epicondyle (section 2), and from 2 to 6 cm below the medial epicondyle (section 3). Fig. 1 shows representative images for the evaluation of ulnar neuropathy in section 2, and the ulnar nerve is indicated with white arrows. (A) Axial MRI on the right side depicts score 0 with a normal finding of the ulnar nerve. (B) Axial T2 fat-suppressed image shows a mild signal change (score 1). (C) Axial T2 fat-suppressed image on the left side presents a severe signal change (score 2). (D) MRI of the right elbow shows a signal change with mild swelling (score 3). (E) Axial MR on the right side depicts severe swelling with a severe signal change (score 4).
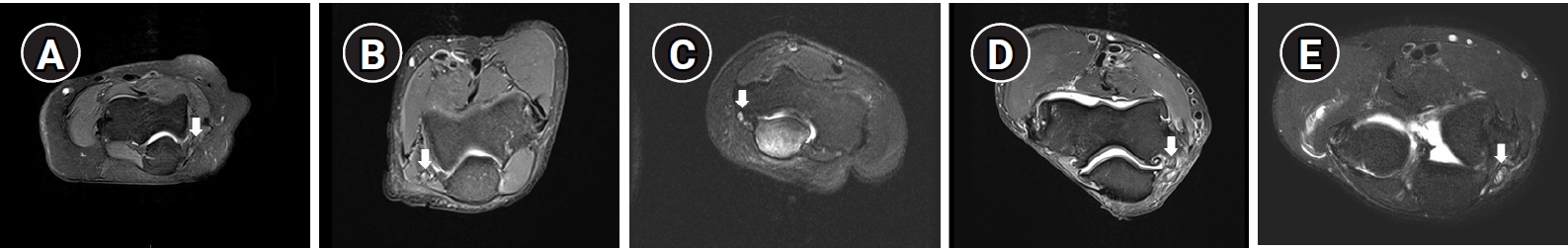
Fig. 2.
The relationships between the severity score on magnetic resonance imaging (MRI) and the parameters of nerve conduction studies. The severity score on MRI showed correlations with (A) the amplitude at the lesion of the segmental study, (B) the conduction velocity at the lesion in the segmental study, (C) the amplitude at the lesion in the inching study, (D) the conduction velocity at the lesion in the inching study, (E) the minimum amplitude of the inching study, and (F) the minimum conduction velocity of the inching study. (G) The severity score on MRI was correlated with the amplitude of the sensory nerve conduction studies.
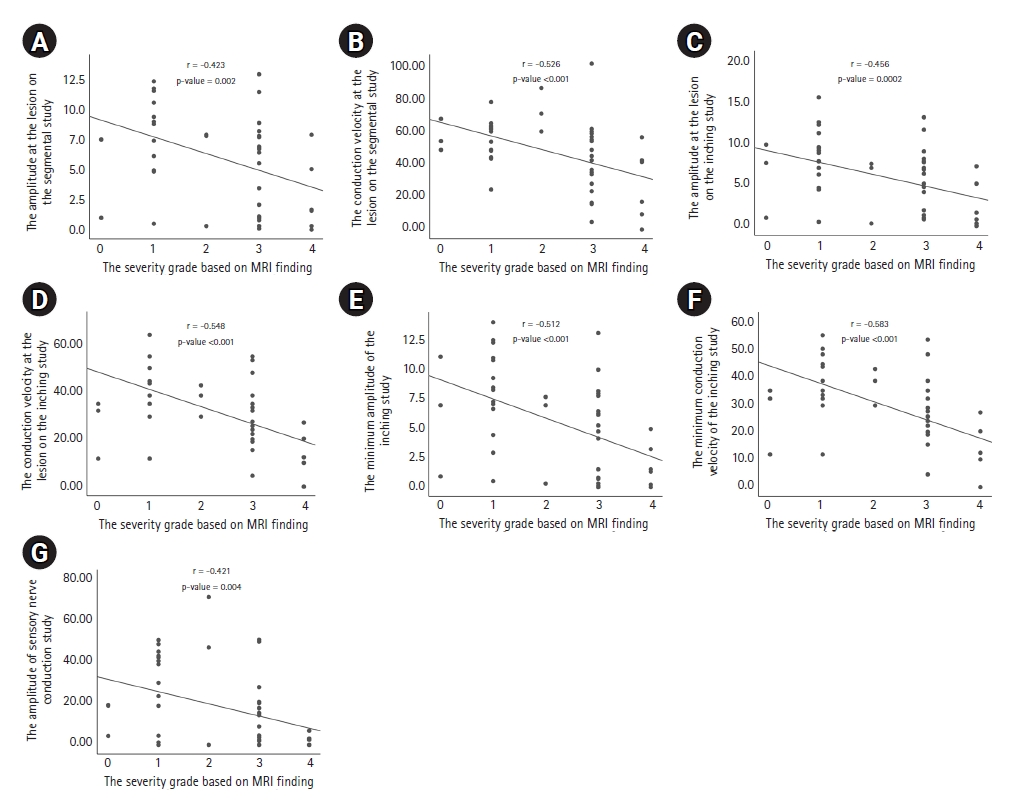
Table 1.
Clinical and Electrophysiologic Findings of 46 Patients with Ulnar Neuropathy at the Elbow
For the age and the duration of the symptoms, the mean ± standard deviation and range are shown, while for the other parameters, number (%) are presented.
FCU, flexor carpi ulnaris; NCS, nerve conduction study; SNAP, sensory nerve action potential; CMAP, compound muscle action potential; MRI, magnetic resonance imaging.
Table 2.
Relationship of the Lesion’s Location Between MRI and Electrodiagnostic Studies in Diagnosing Ulnar Neuropathy at the Elbow (n = 46)
| MRI | Category | NCS | |||
|---|---|---|---|---|---|
| Normal (n = 4) | Abnormal (n = 42) | ||||
| Section 1 | Section 2 | Section 3 | |||
| Normal (n = 3) | 0 | 3 | 0 | ||
| Abnormal (n = 43) | Section 1 | 0 | 0 | 0 | 0 |
| Section 2 | 4 | 3 | 18* | 9 | |
| Section 3 | 0 | 0 | 1 | 2* | |
| Undetermined | 2† | 2† | 2† | ||
Table 3.
The Relationship Between the Severity Score on MRI and the Parameters on Nerve Conduction Study
| Study | Parameter | Spearman correlation coefficient (rho) | p-value |
|---|---|---|---|
| Sensory study | The amplitude of the sensory nerve conduction study | -0.421 | 0.004* |
| Segmental study | The amplitude at the lesion on MRI | -0.423 | 0.002* |
| The conduction velocity at the lesion on MRI | -0.526 | < 0.001* | |
| Inching study | The amplitude at the lesion on MRI | -0.456 | 0.002* |
| The conduction velocity at the lesion on MRI | -0.548 | < 0.001* | |
| The minimum amplitude | -0.512 | < 0.001* | |
| The minimum conduction velocity | -0.583 | < 0.001* |
References
1. Vucic S, Cordato DJ, Yiannikas C, Schwartz RS, Shnier RC: Utility of magnetic resonance imaging in diagnosing ulnar neuropathy at the elbow. Clin Neurophysiol 2006;117:590-595.


2. Azrieli Y, Weimer L, Lovelace R, Gooch C: The utility of segmental nerve conduction studies in ulnar mononeuropathy at the elbow. Muscle Nerve 2003;27:46-50.


3. Landau ME, Campbell WW: Clinical features and electrodiagnosis of ulnar neuropathies. Phys Med Rehabil Clin N Am 2013;24:49-66.


4. Beekman R, Van Der Plas JP, Uitdehaag BM, Schellens RL, Visser LH: Clinical, electrodiagnostic, and sonographic studies in ulnar neuropathy at the elbow. Muscle Nerve 2004;30:202-208.


5. Ayromlou H, Tarzamni MK, Daghighi MH, Pezeshki MZ, Yazdchi M, Sadeghi-Hokmabadi E, et al: Diagnostic value of ultrasonography and magnetic resonance imaging in ulnar neuropathy at the elbow. ISRN Neurol 2012;2012:491892.



6. Pompe SM, Beekman R: Which ultrasonographic measure has the upper hand in ulnar neuropathy at the elbow? Clin Neurophysiol 2013;124:190-196.


7. Keen NN, Chin CT, Engstrom JW, Saloner D, Steinbach LS: Diagnosing ulnar neuropathy at the elbow using magnetic resonance neurography. Skeletal Radiol 2012;41:401-407.


9. Preston DC, Shapiro BE: Ulnar neuropathy at the elbow. In: Preston DC, Shapiro BE, editors. Electromyography and neuromuscular disorders. 3rd ed. Philadelphia: Elsevier Saunders; 2020. p. 372-401.
10. Bäumer P, Dombert T, Staub F, Kaestel T, Bartsch AJ, Heiland S, et al: Ulnar neuropathy at the elbow: MR neurography: nerve T2 signal increase and caliber. Radiology 2011;260:199-206.


11. Campbell WW, Pridgeon RM, Sahni SK: Entrapment neuropathy of the ulnar nerve at its point of exit from the flexor carpi ulnaris muscle. Muscle Nerve 1988;11:467-470.


12. Dumitru D, Amato AA, Zwarts MJ: Focal peripheral neuropathies. In: Dumitru D, Amato AA, Zwarts MJ, editors. Electrodiagnostic medicine. 2nd ed. Philadelphia: Hanley & Belfus; 2002, pp. 1070-1080.
13. Simon NG, Ralph JW, Poncelet AN, Engstrom JW, Chin C, Kliot M: A comparison of ultrasonographic and electrophysiologic ‘inching’ in ulnar neuropathy at the elbow. Clin Neurophysiol 2015;126:391-398.


14. Bielawski M, Hallett M: Motor conduction of ulnar nerve in flexion and extension of elbow in normals and in patients with lesions at the elbow. Muscle Nerve 1982;5:565-566.







